Medical Devices Training
On-Site Training
Training Courses
- Training for Medical Devices
- Minitab Essentials for Medical Devices
- Statistical Quality for Medical Devices
- Factorial Designs (DOE) for Medical Devices
- Workshop
Our Medical Devices series is for professionals working in the Medical Device industry. The course materials include examples with metrics, such as breaking strength, diameter, particle size, nonconformities, and moisture.

MINITAB ESSENTIALS FOR MEDICAL DEVICES
In this 2-day foundational course, you will learn how to efficiently perform data analysis by using Minitab! With Minitab, you can import data, develop sound statistical approaches to explore data, create and interpret compelling graphs, and export results. This course teaches you how to analyze a variety of real-world medical device data sets, which will help you learn how to align your applications with the right statistical tool and interpret statistical output to reveal problems with a process or evidence of an improvement. Learn the fundamentals of important statistical concepts, such as hypothesis testing and confidence intervals, and how to uncover and describe relationships between variables with statistical modeling tools.
This course places a strong emphasis on making sound decisions based upon the practical application of statistical techniques commonly found in the medical device industry.
Topics include:
- Importing and Formatting Data
- Bar Charts
- Histograms
- Boxplots
- Pareto Charts
- Scatterplots
- Tables and Chi-Square Analysis
- Measures of Location and Variation
- t-Tests
- Proportion Tests
- Tests for Equal Variance
- Equivalence tests
- Power and Sample Size
- Correlation
- Simple Linear and Multiple Regression
- One-Way ANOVA
- Multi-Variable ANOVA
Prerequisites: None
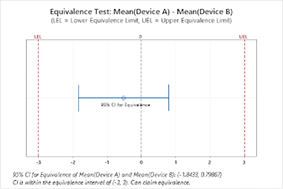
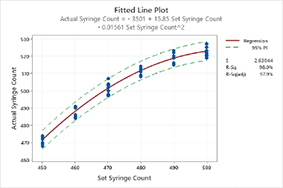

STATISTICAL QUALITY ANALYSIS FOR MEDICAL DEVICES
In this course, you will develop the necessary skills to successfully evaluate and certify measurement systems. Learn the fundamentals of statistical process control and how these important quality tools can provide the necessary evidence to improve and control medical device processes. You will develop the skills to know when and where to use the various types of control charts available in Minitab for your own processes. Plus, learn how to utilize important capability analysis tools to validate your processes relative to internal and customer specifications.
The course emphasis is placed on teaching quality tools as they relate to medical device processes.
Topics include:
- Gage R&R
- Destructive Testing
- Gage Linearity and Bias
- Attribute Agreement
- Variables and Attribute Control Charts
- Capability Analysis for Normal, Nonnormal, and Attribute data
- Acceptance Sampling
Prerequisites: Minitab Essentials for Medical Devices
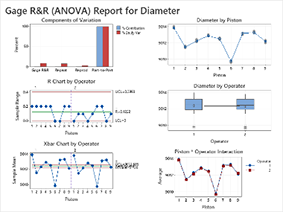
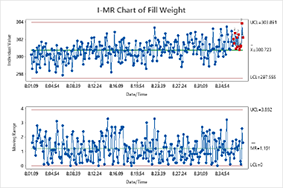

FACTORIAL DESIGNS (DOE) FOR MEDICAL DEVICES
Learn to generate a variety of full and fractional factorial designs using Minitab’s intuitive DOE interface. Real-world medical device applications demonstrate how the concepts of randomization, replication, and blocking form the basis for sound experimentation practices. Develop the skills necessary to correctly analyze the resulting data to effectively and efficiently reach experimental objectives.
Use Minitab’s customizable and powerful graphical displays to interpret and communicate experimental results to improve products and processes, find critical factors that impact important response variables, reduce process variation, and expedite research and development projects.
Topics include:
- Design of Factorial Experiments
- Normal Effects Plot and Pareto of Effects
- Power and Sample Size
- Main Effect, Interaction, and Cube Plots
- Center Points
- Overlaid Contour Plots
- Multiple Response Optimization
Prerequisites: Minitab Essentials for Medical Devices
WORKSHOP
Minitab training provides the foundation for improving your efficiency to use statistics to analyze data. The examples present real-world scenarios to learn the tools, while the exercises allow time to practice. Bring your educational journey full circle by reinforcing the training using data from your company. This affords the attendees the opportunity to relate directly to their own use cases.
The workshop places strong emphasis on making sound decisions based upon the practical application of statistical tools to your company projects with your data.
Topics will be determined by the specific customer data brought to the workshop.
Training Courses
Please contact us if you have any questions about which courses are right for you or to schedule training.